2013
CHEMISTRY
(New Course)
Full Marks: 70
Time: 3 hours
The figures in the margin indicate full marks for the questions
1. Which point defect lowers the density of a crystal? 1
2. Why does the molality of a solution remain unchanged with temperature? 1
3. Write the disproportionation reaction of  1
1
4. Name the compound according to IUPAC rule: 1
OH BR
BR
5. What happens when  1
1

Is ozonolysed?
6. pKb of aniline is more than that of methylamine. Why? 1
7. What types of linkages hold together monomer of DNA? 1
8. Give one example of sulpha drugs. 1
9. 0.52 g of glucose 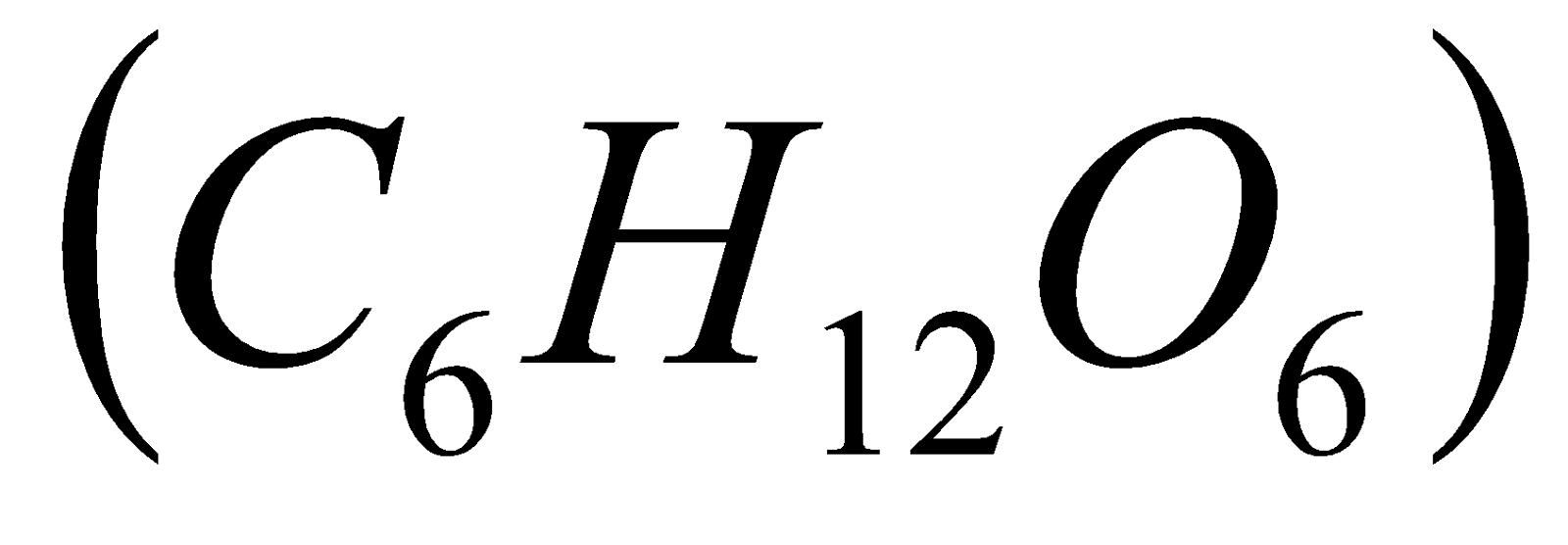 is dissolved in 80.2 g of water. Calculate the boiling point of the solution. (
is dissolved in 80.2 g of water. Calculate the boiling point of the solution. ( for water is 0.52 K kg
for water is 0.52 K kg ) 2
) 2
10. Define osmotic pressure. How can molar mass of a substance be determined from the measurement of osmotic pressure of a solution? 2
11. The rate constant of a reaction at 500 K and 700 K are  and
and 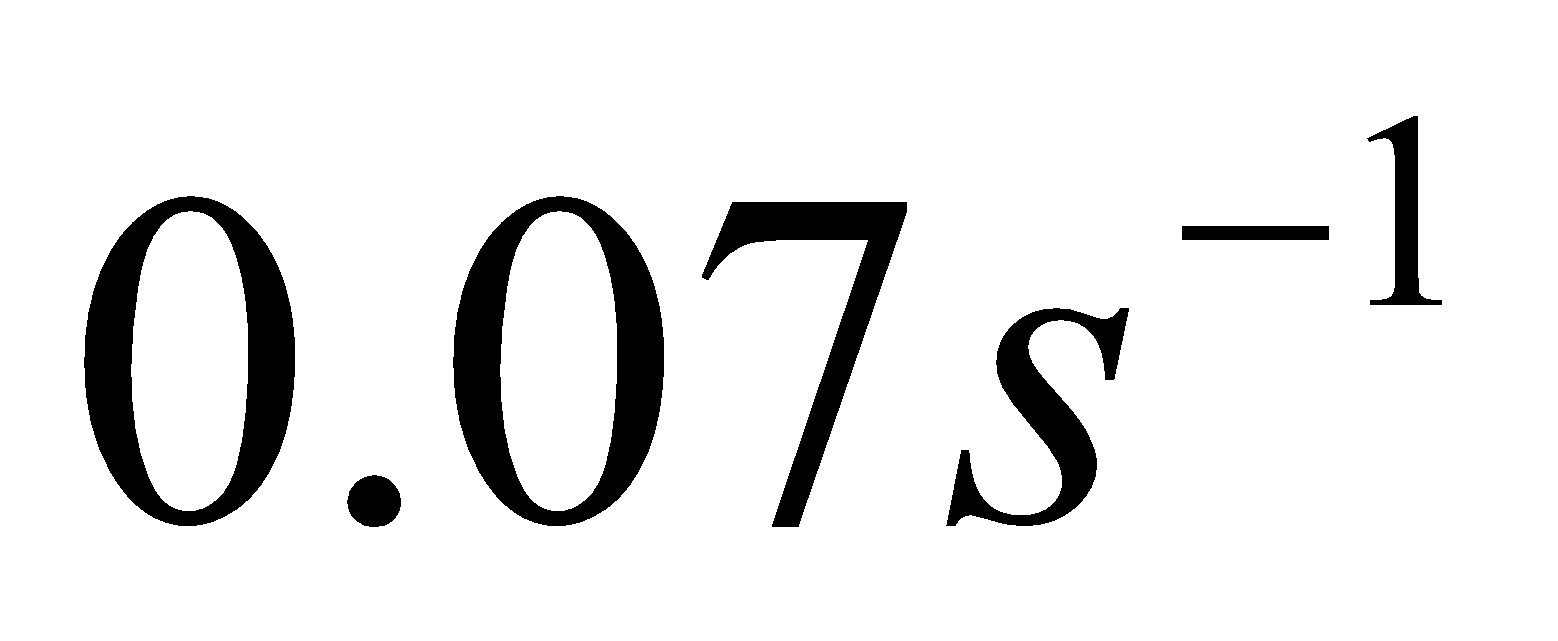 respectively. Calculate the value of activation energy for the reaction.
respectively. Calculate the value of activation energy for the reaction. 
Or
For a chemical reaction variation in concentration, In [R] vs. time (min) plot is shown below: 2
In [R]
T (min)
- What is the order of the reaction?
- What is the unit of rate constant k, for the reaction?
- If initial concentration of the reactant is half of the original concentration, how will t
change?
- Draw the plot of log
vs. time (s).
12. Mention any two factors which distinguish physisorption from chemisorptions. 2
13. (a) What is observed when a beam of light is passed through a colloidal solution? 1
(b) What are lyophobic colloids? Give one example. 1
14. Explain the bleaching action of . 2
. 2
Or
15. How will you convert the following? Give chemical equations only. 2
- Benzene to phenol.
- Aniline to phenylisocyanide.
16. (a) Complete the following reaction: 1
(b) An alkyl chloride (X) reacts with magnesium metal in presence of dry either followed by treatment of ethanol gives propane. Write the structure of the alkyl chloride (X). 1
17. (a) Give one chemical test to distinguish between the following pair: 1
Pentan-2one and Pentan-3-one
(b) Identify A and B: 1
18. Justify the following:
- Sleeping pills are recommended to patient suffering from sleeplessness but it is not advisable to take them without consulting the doctor. 1
- Why do we require artificial sweetening agents? 1
19. (a) What is semiconductor? Mention the two main types of semiconductor. 1
(b) Sodium crystallizes in a body-centred cubic (bcc) unit cell. Calculate the approximate number of unit cells in 9.2 g of sodium. (Atomic mass of Na = 23 u) 2
Or
(a) Mention the types of semiconductor, (n-type or p-type) when silicon doped with phosphorus. 1
(b) Gold metal crystallizes in a face-centred cubic unit cell (fcc). Determine the density of gold. (Atomic mass of gold = 179 u, atomic radius = 0.144 nm,  ] 2
] 2
20. For the reaction 
The following results have been obtained:
SL. No.
|
Rate of disappearance of
| |
1
| ||
2
| ||
3
|
- Calculate order of the reaction.
- Write rate law.
- Calculate rate constant of the reaction. 3
21. Describe the role of the following in the processes mentioned:
- NaCN in the extraction of silver from silver ore. 1
- Limestone in the metallurgy of iron. 1
- Iodine in the refining of zirconium. 1
Or
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron. 3
22. (a) Give reasons: 1+1=2
Is coloured while
is colourless.
is a high spin complex iron.
(b) Draw the two geometrical isomers of the complex compound . 1
. 1
Or
Define the following terms with one example each: 3
- Coordination sphere.
- Coordination number.
- Ligands.
23. What happens, when –
- Ethanal is treated with methyl magnesium bromide and the product is hydrolysed;
- Phenol is heated with zinc dust;
- Methoxyethane is treated with excess HI. 1+1+1=3
24. An organic compound contains 69.77% carbon, 11.63% of hydrogen and the rest is oxygen. The molecular mass of the compound is 86 u. The compound does not reduce Tollens reagent but reacts with Brady’s reagent to give yellow precipitate. On vigorous oxidation the molecule produces ethanoic acid and propanoic acid. The compound also shows iodoform test. Identify and name the compound, and write the reactions. 3
Or
An organic compound  on hydrolysis with strong aqueous acid gives another compound B which is a monobasic aromatic carboxylic acid. The compound B on treatment with ammonia gives a salt which on heating Gives C. The compound C undergoes Hofmann’s bromamide reaction to yield aniline. Name A, B and C and write the chemical reactions involved. 3
on hydrolysis with strong aqueous acid gives another compound B which is a monobasic aromatic carboxylic acid. The compound B on treatment with ammonia gives a salt which on heating Gives C. The compound C undergoes Hofmann’s bromamide reaction to yield aniline. Name A, B and C and write the chemical reactions involved. 3
25. (a) Identify A, B, C and D: 2
(b) Write one chemical test to distinguish between ethylamine and aniline. 1
26. (a) Name the vitamin whose deficiency causes rickets. 1
(b) Define the following terms in relation to protein: 2
- Peptide linkage.
- Denaturation.
Or
Name the four bases present in DNA. Which one of these is not present in RNA? 3
27. (a) Name the monomers of Bakelite. 1
(b) What is the primary feature necessary for a monomer to make it useful in a condensation polymerization reaction? 1
(c) What is meant by copolymerization? Give one example of a copolymer. 1
28. (a) State Kohlrausch law of independent migration of ions. 1
(b) What is primary battery? Give one example. 1
(c) Three electrolytic cells A, B and C containing electrolytes  respectively were connected in series. A steady current of 1.5 A was passed through them. 1.45 g Ag were deposited at the cathode of cell B.
respectively were connected in series. A steady current of 1.5 A was passed through them. 1.45 g Ag were deposited at the cathode of cell B.
- How long did the current flow?
- What mass of copper and zinc were deposited?
(Atomic mass of Cu = 63.5 u, Zn = 65.3 u, Ag = 108 u) 3
Or
- How do you explain with the help of graph, the increase in the value of molar conductivity with dilution in case of strong and weak electrolyte? 2
- Calculate the e.m.f. of the cell at 298 K, in which the following reaction takes place: 3
[Given that ]
]
29. Answer the following: 1x5=5
- Ozone acts as a powerful oxidizing agent. Give reason. 1
- Compute the following reaction: 1
- Which reaction was used by Bartlett to prepare the first noble gas compound? 1
Is known but
is not known. Give reason. 1
- Bismuth is a strong oxidizing agent in the pentavalent state [O.N. = 5]. Give reason. 1
Or
- Complete the following chemical reaction equations: 3
- Draw the structure of the following molecules and mention their shapes: 2
30. Give reasons:
- Why are Zn, Cd and Hg normally not regarded as transition metals? 1
- Why is first ionization enthalpy of Cu is higher than that of Na? 1
- Name one ore each of manganese and chromium. 1
- Why is HCI not used to acidify a permanganate solution in volumetric estimation of
or
? 1
- What is lanthanoid contraction? 1
Or
- How would you account for the following? 3
- Transition metals and many of their compounds show paramagnetic behaviour.
- The enthalpies of atomization of the transition metals are high.
- The transition metal compounds are good catalyst.
- How potassium permanganate is prepared? Give necessary chemical equations. 2
