2014
CHEMISTRY
(New Course)
Full Marks: 70
Time: 3 hours
The figures in the margin indicate full marks for the questions
1. How much H2 in grams will be liberated if 1F electricity is passed through acidified water? 1
2. Why does physisorption decrease with the increase of temperature? 1
3. Complete the following reaction: 1
4. Arrange the following in order of decreasing bond dissociation enthalpy. 1
5. Write the structural formula of the following compound: 1-Bromobut-2-ene. 1
6. Give reason for the higher boiling point of ethanol in comparison to Methoxyethane. 1
7. Which one of the following compounds would undergo Hell-Volhard-Zelinsky reaction and why? 1
- Benzoic acid.
- Propanoic acid.
8. What is the difference between nucleoside and nucleotide? 1
9. X-ray diffraction studies show that copper crystallizes in a fee lattice with edge length of  If density of copper is
If density of copper is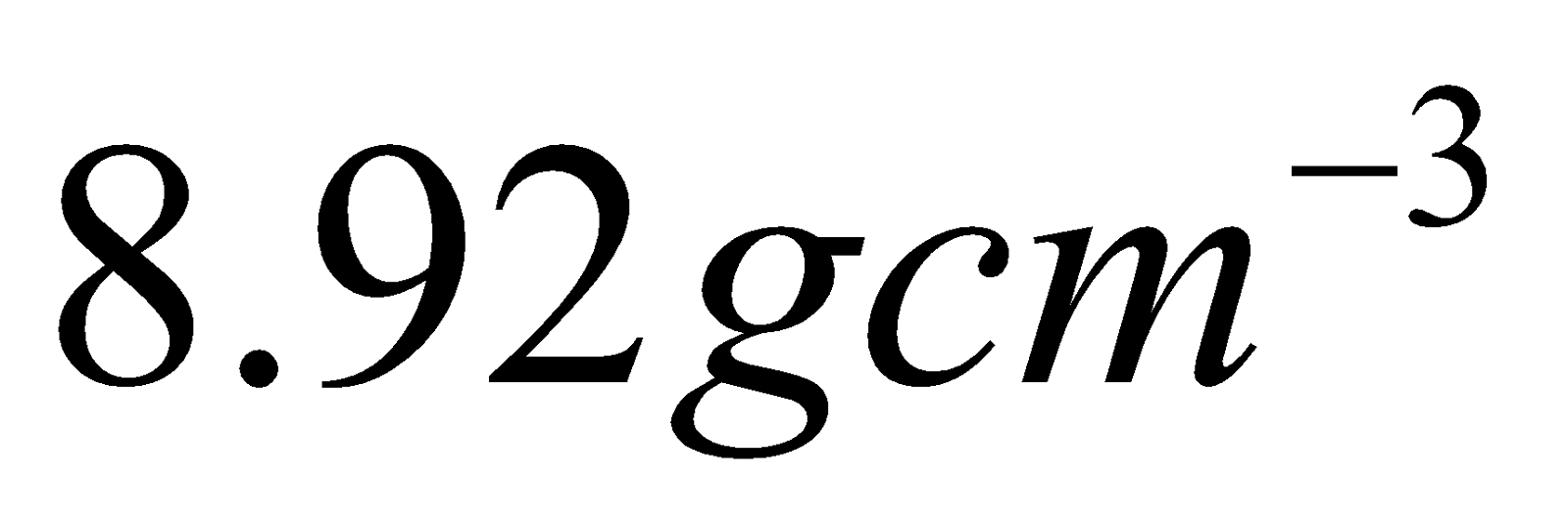 , calculate the atomic mass of copper. 2
, calculate the atomic mass of copper. 2
10. Explain the following: 1+1=2
- Frenkel defect.
- Ferromagnetism.
11. Copper does not dissolve in HCL while it does dissolve in HNO3. Explain. 2
12. (a) Depict the galvanic cell in which the following reaction takes place: 1
(b) Write the Nernst equation of the following cell: 1
13. Show that for a first order reaction, the half life is independent of the initial concentration of reactants. 2
14. Describe a method of refining nickel. 2
Or
Differentiate between “minerals” and “ores”. 2
15. (a) What are interhalogen compounds? 1
(b) Write the balanced chemical equation for the reaction between sulphur dioxide and chorine in presence of charcoal as catalyst. 1
16. Why is 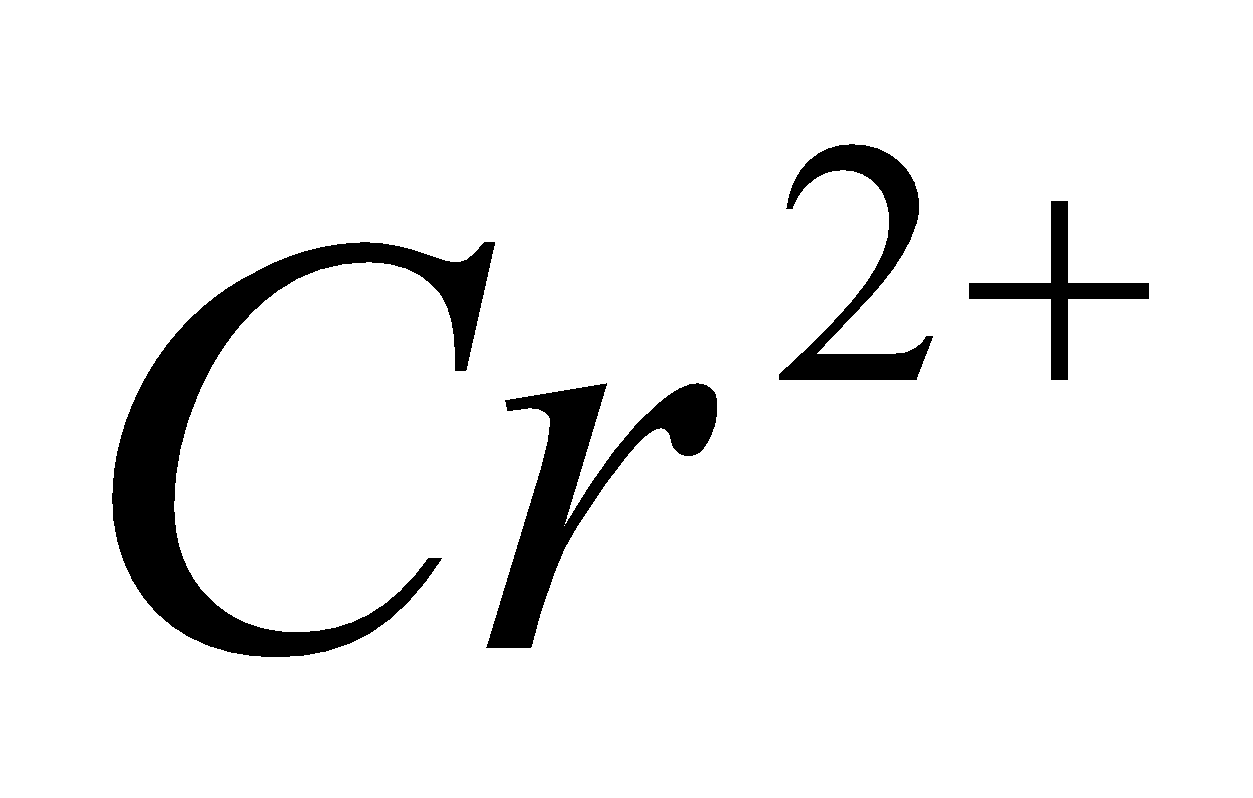 reducing and
reducing and oxidizing when both having
oxidizing when both having  configuration? 2
configuration? 2
17. An aromatic compound (A) on treatment with aqueous ammonia and heating forms a compound (B) which on heating with  and KOH forms a compound (C) of molecular formula
and KOH forms a compound (C) of molecular formula . Write the structures and IUPAC names of compounds A, B and C. 2
. Write the structures and IUPAC names of compounds A, B and C. 2
18. Methanamine can be prepared using Gabriel phthalimide synthesis. Write chemical equation only for the synthesis. 2
Or
How can you convert aniline to p-nitroaniline? Give the chemical equations only. 2
19. (a) Identify the reaction order from each of the following rate constants. 1
(b) The conversion of molecule A to B follows second order kinetics. If concentration of A is increased four times how will the rate of formation of B be affected? 1
(c) Define collision frequency. 1
20. Answer either (a) or (b):
(a) (i) What are promoters. 1
(ii) Describe the cleansing action of soaps. 2
Or
(b) Explain the terms: 1+1+1=3
(i) Electrophoresis.
(ii) Dialysis.
(iii) Aerosol.
21. Answer either (a) or (b):
(a) (i) Actinoid contraction is greater from element to element than lanthanide contraction. Why? 1
(ii) What are interstitial compounds? 1
(iii) Calculate the number of unpaired electrons in the gaseous ion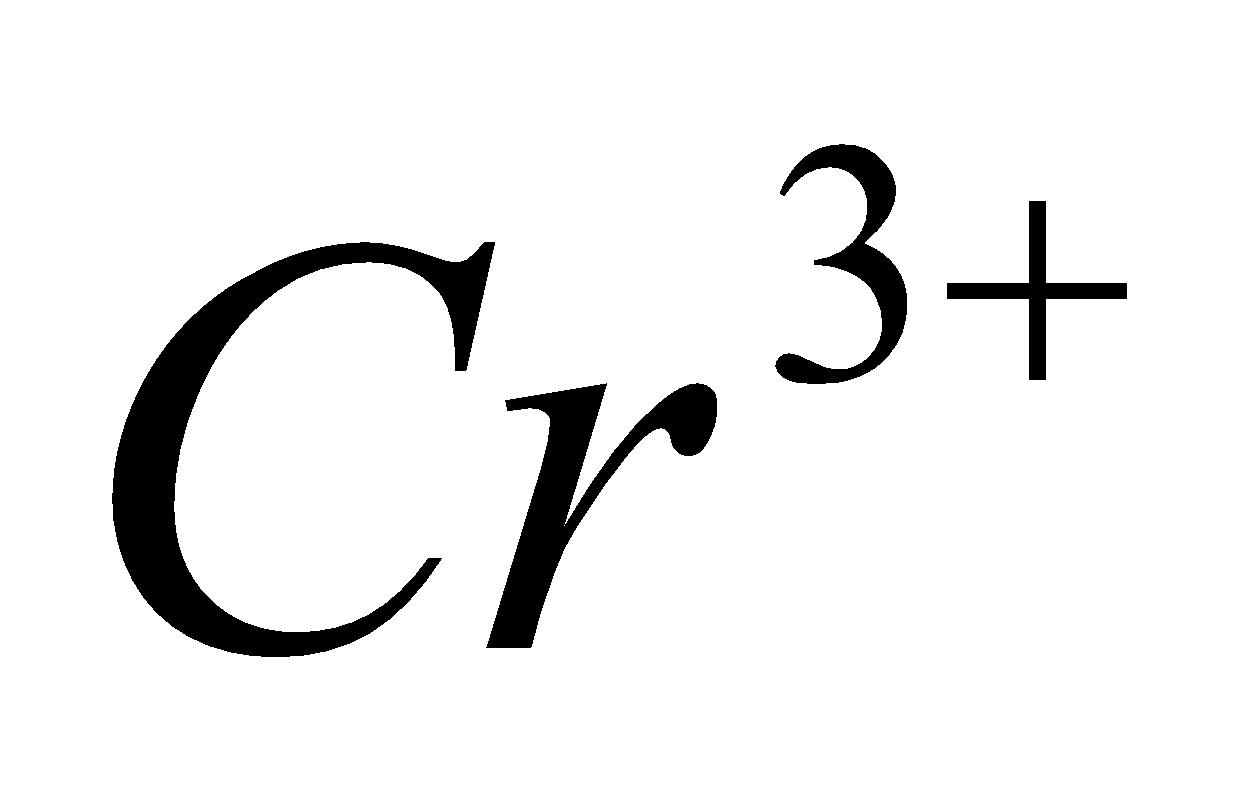 . 1
. 1
Or
(b) How does the acidified permanganate solution react with the following? Write the ionic equations for the reactions. 3
(i) KI.
(ii) iron (II) ion.
(iii) Oxalic acid.
22. (a) Write the IUPAC name of – 1
(b) Draw figure to show the splitting of d-orbital’s in an octahedral crystal field. 1
(c) Write the structures of the geometrical isomers of the compound  1
1
23. Answer either (a), (b) and (c) or (d) and (e):
- Explain why Grignard reagents should be prepared under anhydrous condition. 1
- Which of the following compound would undergo SN1 reaction faster and why? 1
- CI II.
CI
- What are ambident nucleophiles? Give an example. 1
Or
- Write the mechanism of the following reaction: 2
- How can you convert benzene to diphenyl? 1
24. Complete the following reactions: 3
25. (a) What are reducing sugars? 1
(b) What is the basic structural difference between starch and cellulose? 1
(c) What are essential and non-essential amino acids? 1
26. (a) Classify the following as addition polymer or condensation polymer. 1
- Teflon.
- Bakelite.
(b) Write the names and structural formulae of monomers of Dacron. 1
(c) Define thermoplastic polymers.
27. (a) Write what is meant by broad spectrum antibiotics. 1
(b) Write how antiseptics differ from disinfectants. 1
(c) Identify one analgesic and one antacid from the following: 1
Aspirin, Chloramphenicol, Cimetidine, Sulphanilamide
28. Answer either (a) or (b):
(a) (i) Calculate the molarity of a solution containing 1.0g of in 250ml. solution. 2
in 250ml. solution. 2
(ii) What is meant by positive deviation from Raoult’s Law? Explain why this deviation is observed. 2
(iii) State Henry’s law. 1
Or
(b) (i) 200 of an aqueous solution of a protein contains 1.26g of the protein. The osmotic pressure of such a solution at 300K is found to be
of an aqueous solution of a protein contains 1.26g of the protein. The osmotic pressure of such a solution at 300K is found to be 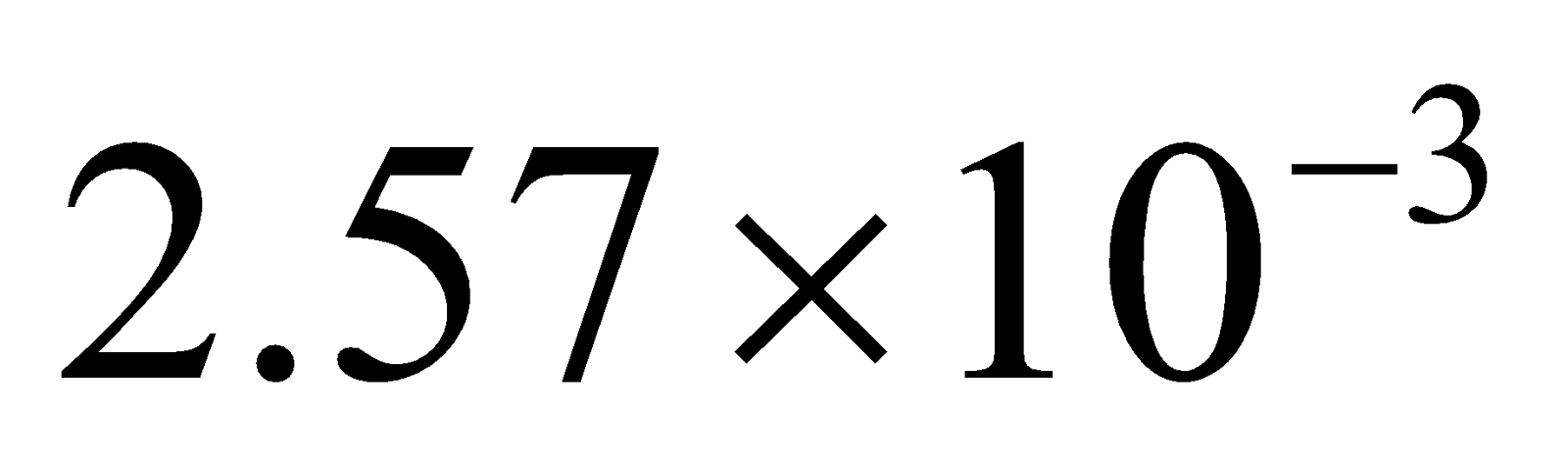 bar. Calculate the molar mass of the protein. 2
bar. Calculate the molar mass of the protein. 2
(ii) State Raoult’s law. 1
(iii) Name a method for desalination of sea water. 1
(iv) What are azeotropes? 1
29. Answer either (a) and (b) or (c) (d) and (e)
- Complete the following chemical equations: 3
- Nitrogen exists as diatomic molecule and phosphorous as
. Why? 2
Or
- Name an aerosol that depletes ozone. 1
- Account for the following: 2
- Halogens are coloured.
- Noble gases have very low boiling points.
- Complete the following chemical equation: 2
30. (a) Write one chemical test to distinguish between propanal and propanone. 1
(b) Write why aldehydes are generally more reactive than ketones in nucleophilic addition reactions. 2
(c) Predict the products of the following reactions: 2
